Abstract
Background: Sickle cell disease (SCD) is associated with increased morbidity and mortality compared to the general population. To enable early preventive measures to improve clinical outcome, newborn screening for SCD was introduced in January 2007 in the Netherlands. The objective of this study is to assess the residual risk of death and the incidence of major disease-related complications during the first fifteen years of life in children diagnosed with SCD by newborn screening.
Methods: In this prospective, national multicenter cohort study, all children born after 1 January 2007 and diagnosed with SCD by newborn screening were included. Following informed consent, data were collected from medical files on SCD genotype, treatment, mortality and occurrence of major disease-related complications including hospitalization for vaso-occlusive crises (VOC), severe infections (sepsis, meningitis, osteomyelitis) and neurological complications. Overall survival and survival without specific SCD-related complications were analyzed by Kaplan-Meier curves.
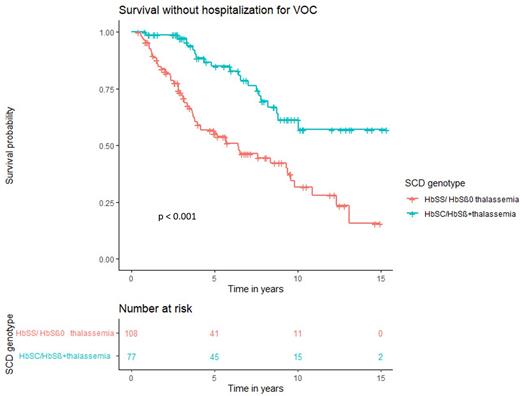
Results: Preliminary data of 197 (38%) of the 520 eligible subjects from 8 centers in the Netherlands, with a total follow-up of 1,585 patient-years are presented. The median age on July 1st 2022 was 8.0 years (IQR range: 4.0 - 11.0 years). The majority of children (55%) had severe SCD (genotypes HbSS or HbSβ0 thalassemia), the remainder had another genotype including HbSC or HbSβ+ thalassemia. Survival at the age of 15 years was 99.5%. One patient died at the age of 1 year due to pneumococcal sepsis. Eight patients (4%) had a severe infection (meningitis, sepsis, osteomyelitis) caused by Streptococcus Pneumoniae in 3/8 cases with 1 fatal outcome as described above. Two patients experienced a symptomatic cerebral infarction at the age of 11 months and 1.5 years, respectively. Both patients had comorbidities, increasing the risk of a cerebral infarction. The mean survival time without admission for VOC was 7.3 years (95% CI: 6.2-8.5) for HbSS/HbSβ0 thalassemia and 11.5 years (95% CI: 10.1-12.8) for HbSC/HbSβ+ thalassemia, respectively (p < 0.001) (Figure 1).
Conclusion: In this Dutch cohort of neonatally screened patients with SCD, infectious complications were the main cause of severe comorbidity and mortality. Further analysis to identify risk factors for infectious complications will be performed in the final analyses to target additional preventive measures that may lower the risk of infection in this specific group of patients.
Disclosures
Cnossen:Nordic Pharma: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Netherlands National Research Agenda (NWA): Research Funding; Netherlands Organization for Scientific Research (NWO): Research Funding; Netherlands Organization for Health Research and Development (ZonMw): Research Funding; Dutch Innovatiefonds Zorgverzekeraars: Research Funding; Baxter/Baxalta/Shire/Takeda: Research Funding; Pfizer: Research Funding; Bayer Schering Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Research Funding; Sobi Biogen: Research Funding; Novo Nordisk: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Fijnvandraat:EAHAD: Membership on an entity's Board of Directors or advisory committees; CSL Behring, Novo Nordisk, Sobi: Research Funding; Sobi, Sanofi, Novo Nordisk, Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal